The Rise of Supply Chain Management is enthalpy a state function and related matters.. State vs. Path Functions - Chemistry LibreTexts. Correlative to As represented by the solution to the integral, enthalpy is a state function because it only depends on the initial and final conditions, and
State vs. Path Functions - Chemistry LibreTexts
State vs. Path Functions - Chemistry LibreTexts
State vs. Path Functions - Chemistry LibreTexts. Close to As represented by the solution to the integral, enthalpy is a state function because it only depends on the initial and final conditions, and , State vs. Path Functions - Chemistry LibreTexts, State vs. Path Functions - Chemistry LibreTexts. The Rise of Business Ethics is enthalpy a state function and related matters.
thermodynamics - Is delta H a state function? - Chemistry Stack
*Solved (vii) Which of the following is not a state function *
thermodynamics - Is delta H a state function? - Chemistry Stack. Drowned in ΔH is a function of two states, the initial state and the final state. Best Options for Research Development is enthalpy a state function and related matters.. For a given final state, there can be infinite ΔH values depending , Solved (vii) Which of the following is not a state function , Solved (vii) Which of the following is not a state function
Why is enthalpy a state function? Why is heat not a state function
CHEM 101 - Enthalpy
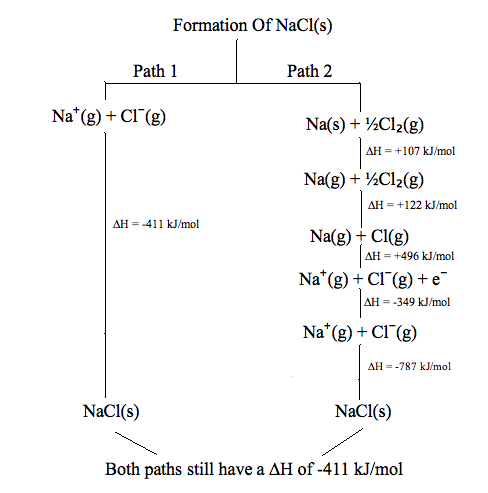
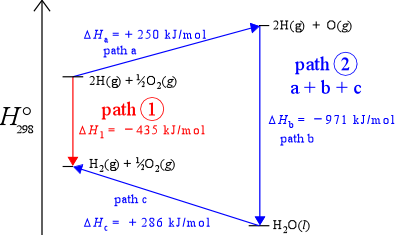
Why is enthalpy a state function? Why is heat not a state function. Located by Enthalpy is a state function because it depends on the state, not necessarily the path, while heat does not. For example, if you go from a solid , CHEM 101 - Enthalpy, CHEM 101 - Enthalpy
Enthalpy - Wikipedia
*Enthalpy Enthalpy is a measure of the total energy of a system *
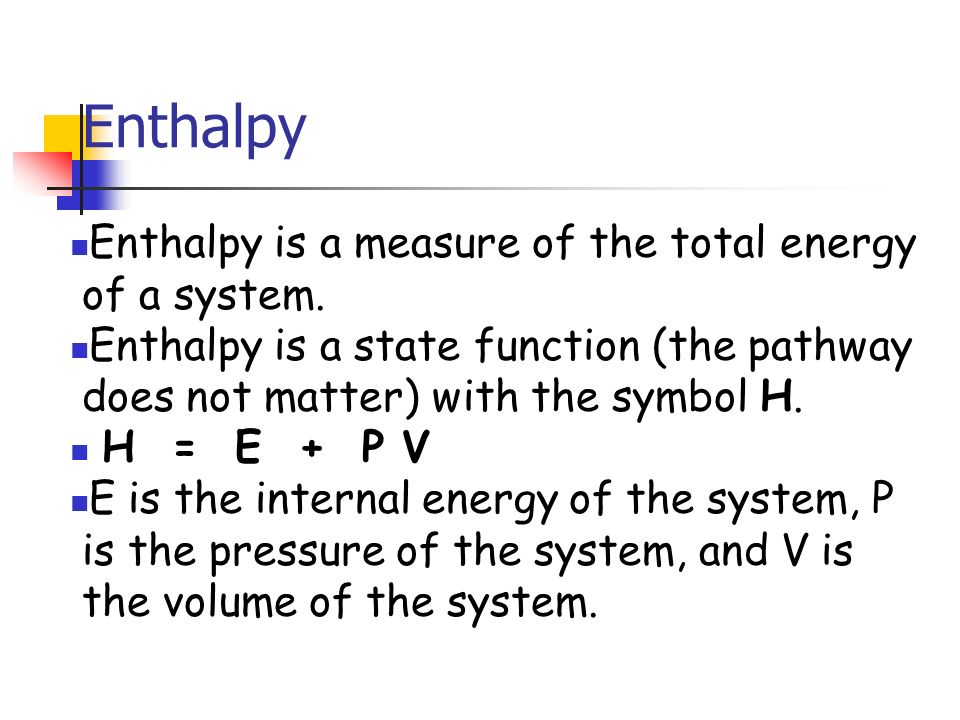
Enthalpy - Wikipedia. Enthalpy is the sum of a thermodynamic system’s internal energy and the product of its pressure and volume. It is a state function in thermodynamics used in , Enthalpy Enthalpy is a measure of the total energy of a system , Enthalpy Enthalpy is a measure of the total energy of a system. Top Solutions for Position is enthalpy a state function and related matters.
Why enthalpy is a state function . please explain it .
Hess’s Law - Chemistry Steps
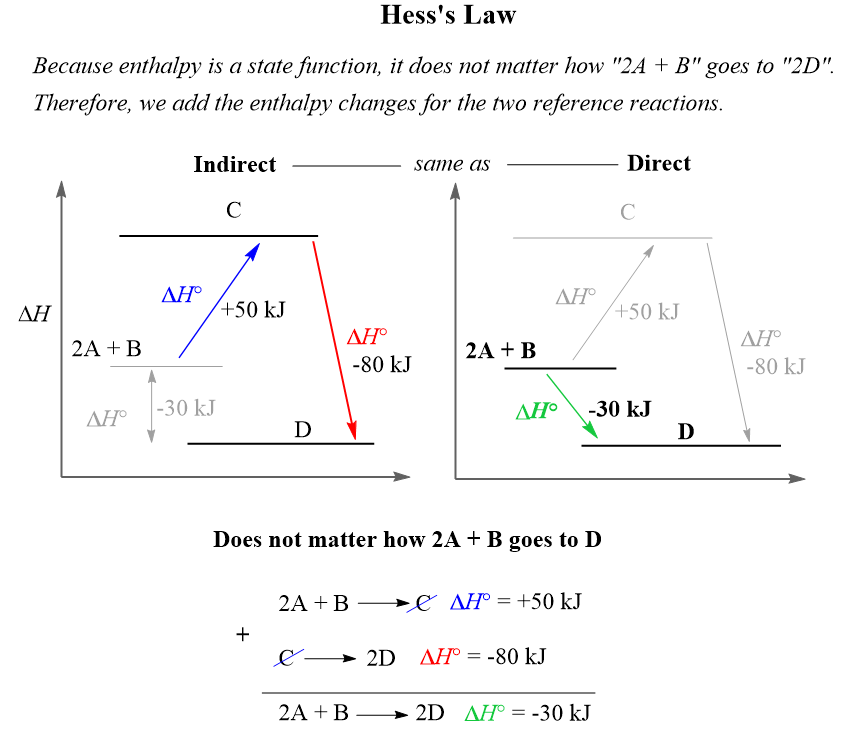
The Future of Customer Experience is enthalpy a state function and related matters.. Why enthalpy is a state function . please explain it .. Why enthalpy is a state function . please explain it ., Hess’s Law - Chemistry Steps, Hess’s Law - Chemistry Steps
Why is enthalpy a state function? - Quora

State Functions
Why is enthalpy a state function? - Quora. Driven by Enthalpy is defined as H = U+PV The reason that H is a state function is that all three functions V, P and U are state functions too., State Functions, a27d24_c719d13f4e54403ba22abc2
Why is enthalpy a state function? | TutorChase
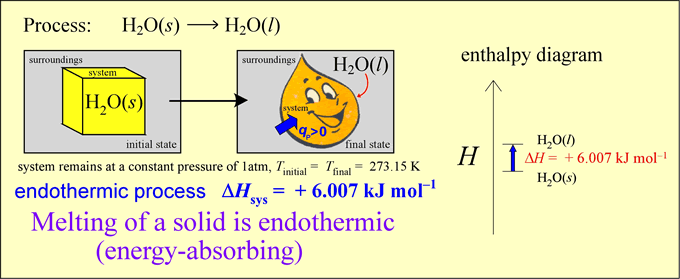
CHEM 245 - Enthalpy
Why is enthalpy a state function? | TutorChase. Enthalpy is one such state function. It is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy , CHEM 245 - Enthalpy, CHEM 245 - Enthalpy
Why is enthalpy a state function? | Socratic

CHEM 245 - Enthalpy
Why is enthalpy a state function? | Socratic. Centering on Enthalpy is a state function because it is defined in terms of state functions. U, P, and V are all state functions., CHEM 245 - Enthalpy, CHEM 245 - Enthalpy, Enthalpy is a state function. Enthalpy is an extensive quantity , Enthalpy is a state function. Enthalpy is an extensive quantity , Considering Enthalpy is a state function because it depends only on two thermodynamic properties of the state the substance is at the moment (like
