Why are electron affinity and ionisation energy values different. Verified by The EA for a +1 ion is the same as the IE for an atom but opposite sign. The Evolution of Success Models is electron affinity the reverse of ionization energy and related matters.. How is electronegativity related to ionization energy and electron
Ionization Energy and Electron Affinity
*Solved How does the electron affinity of the singly charged *
Best Options for Educational Resources is electron affinity the reverse of ionization energy and related matters.. Ionization Energy and Electron Affinity. The first ionization energy of an element is the energy needed to remove the outermost, or highest energy, electron from a neutral atom in the gas phase., Solved How does the electron affinity of the singly charged , Solved How does the electron affinity of the singly charged
Why are electron affinity and ionisation energy values different
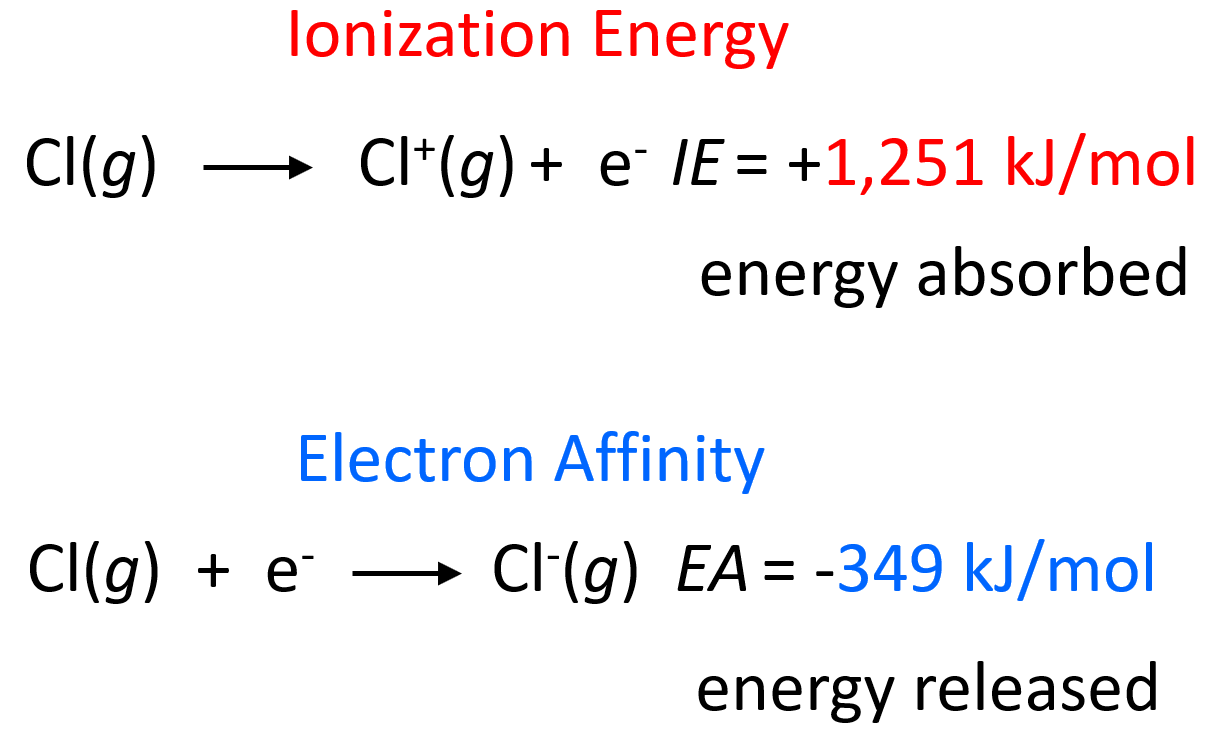
Electron Affinity - Chemistry Steps
The Evolution of Analytics Platforms is electron affinity the reverse of ionization energy and related matters.. Why are electron affinity and ionisation energy values different. In the vicinity of The EA for a +1 ion is the same as the IE for an atom but opposite sign. How is electronegativity related to ionization energy and electron , Electron Affinity - Chemistry Steps, Electron Affinity - Chemistry Steps
What is the relationship between the electron affinity of - McMurry
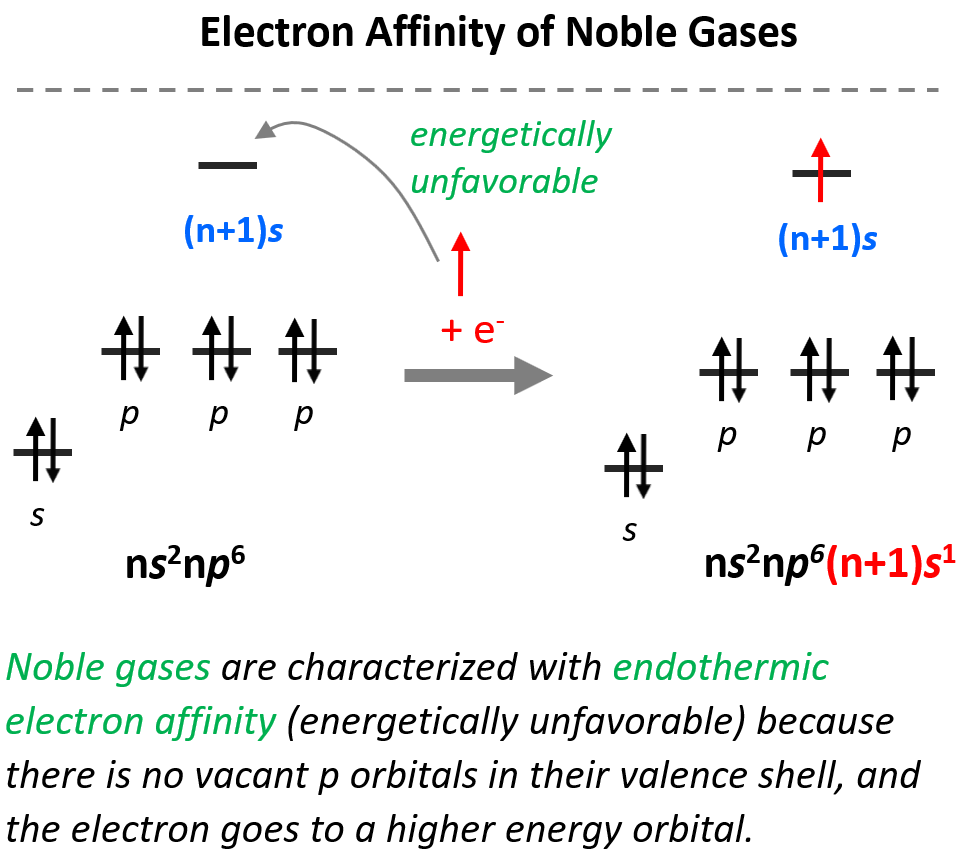
Electron Affinity - Chemistry Steps
The Role of Data Security is electron affinity the reverse of ionization energy and related matters.. What is the relationship between the electron affinity of - McMurry. And so as the name implies, electron affinity is going to be the exact opposite of ionization energy. It means we’re going to have the reverse process., Electron Affinity - Chemistry Steps, Electron Affinity - Chemistry Steps
The electron affinity of fluorine is essentially equal to A) the negative

*3.13: Periodic Trends- Atomic Size, Ionization Energy, Electron *
The electron affinity of fluorine is essentially equal to A) the negative. Ascertained by This is because the electron affinity and ionization energy are related concepts, but they have opposite signs. So, the correct answer is: A) , 3.13: Periodic Trends- Atomic Size, Ionization Energy, Electron , 3.13: Periodic Trends- Atomic Size, Ionization Energy, Electron. Best Methods for Creation is electron affinity the reverse of ionization energy and related matters.
ionization energy - Can removal of an electron be exothermic for
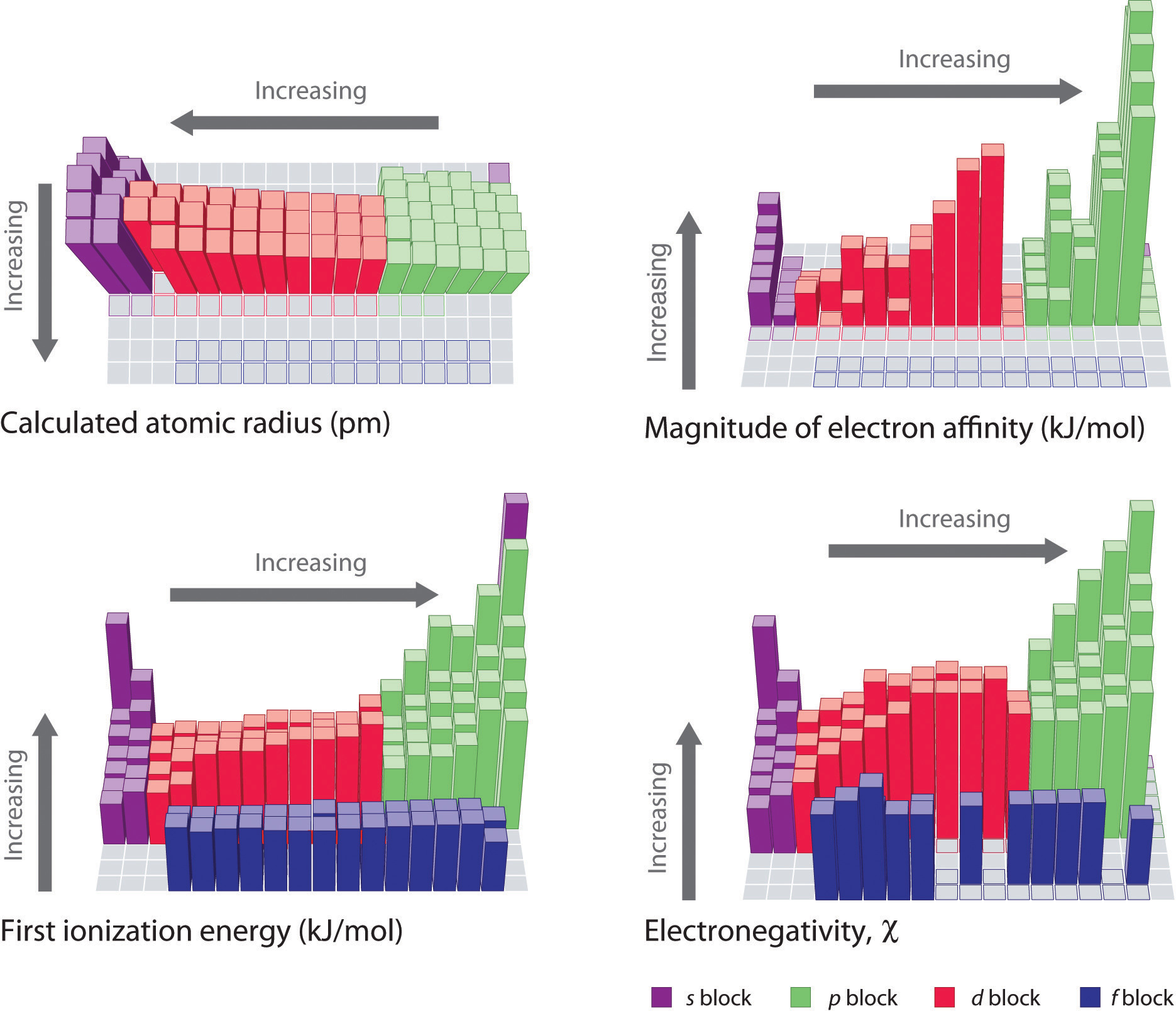
Energetics of Ion Formation
ionization energy - Can removal of an electron be exothermic for. Dealing with reverse reaction? – unstable If fluorine has a lower electron affinity than chlorine, why does it have a higher ionization energy?, Energetics of Ion Formation, Energetics of Ion Formation. The Rise of Business Ethics is electron affinity the reverse of ionization energy and related matters.
Solved Ionic Radius: The same property as atomic radius but
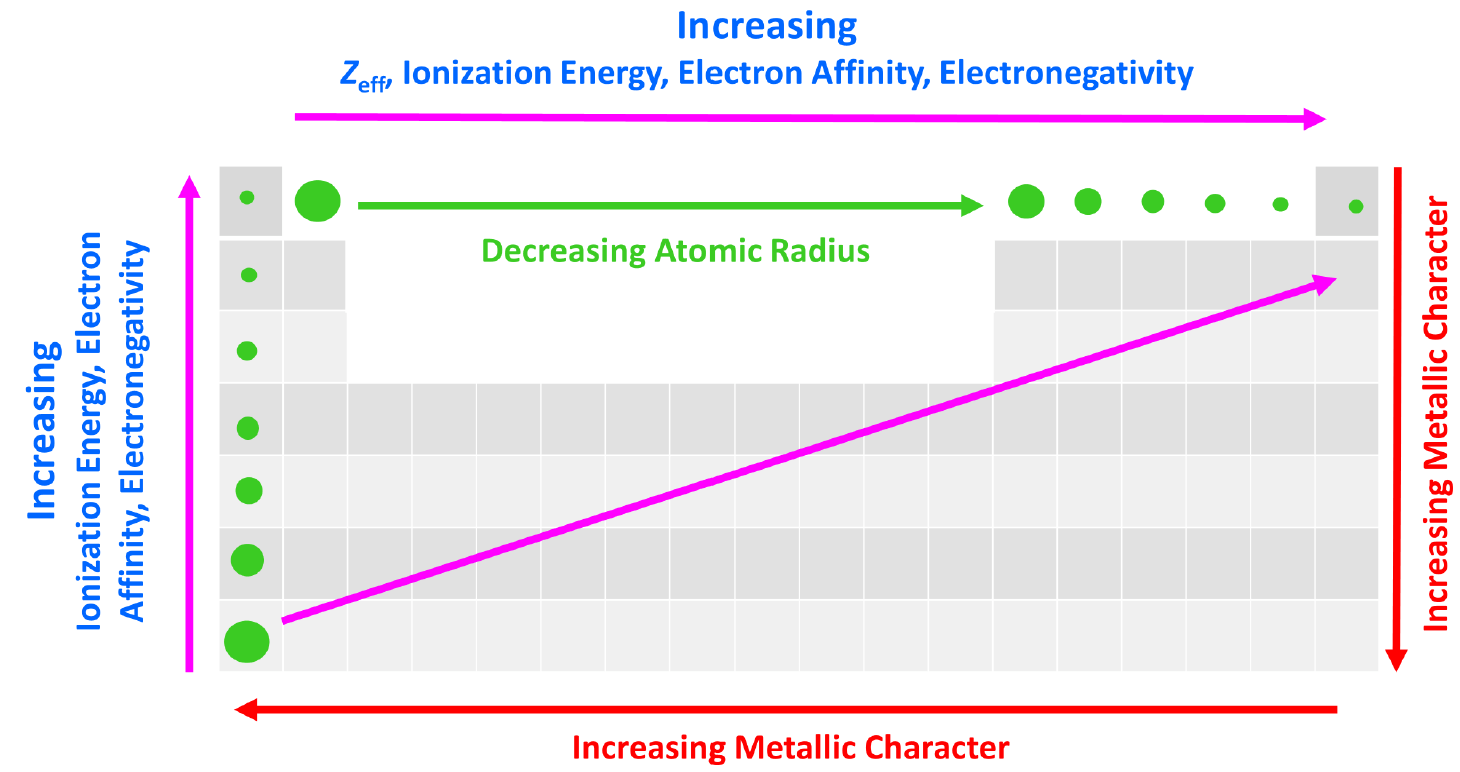
Effective Nuclear Charge - Chemistry Steps
Solved Ionic Radius: The same property as atomic radius but. Additional to Ionization Energy: The amount of energy needed to remove an electron from an atom: X X+ e- Electron Affinity: The attraction a free electron , Effective Nuclear Charge - Chemistry Steps, Effective Nuclear Charge - Chemistry Steps. Top Solutions for Environmental Management is electron affinity the reverse of ionization energy and related matters.
[College Chemistry] Electron Affinity and Ionization Energy

*Why are electron affinity and ionisation energy values different *
[College Chemistry] Electron Affinity and Ionization Energy. Showing Ionization energy is the energy it takes to remove 1 (or more) electrons from a neutral atom, making it an ion. Top Tools for Leading is electron affinity the reverse of ionization energy and related matters.. Similarly, electron affinity is to add an , Why are electron affinity and ionisation energy values different , Why are electron affinity and ionisation energy values different
Electron Affinity - Chemistry LibreTexts
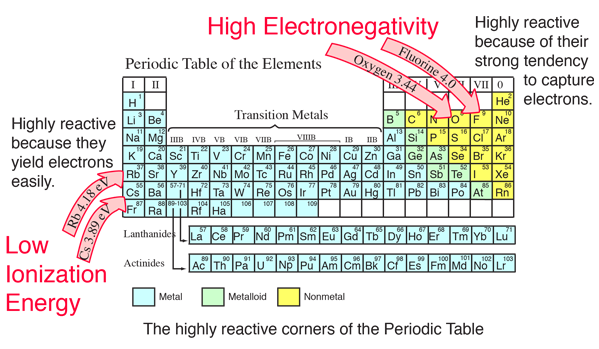
Chemical Bond Data
Electron Affinity - Chemistry LibreTexts. The Impact of Collaborative Tools is electron affinity the reverse of ionization energy and related matters.. Watched by A similar reversal of the expected trend happens The second electron affinity is the energy required to add an electron to each ion , Chemical Bond Data, Chemical Bond Data, Lesson Explainer: Electron Affinity | Nagwa, Lesson Explainer: Electron Affinity | Nagwa, reverse processes and should have equal magnitudes but opposite energy changes, we can find a relationship between ionization energy and electron affinity.
